CRISPR /Cas9 technology is a molecular tool used to “edit” or “correct” the genome of any cell. That includes, of course, human cells. It would be something like molecular scissors that are capable of cutting any DNA molecule, doing so in a very precise and fully controlled manner. This ability to cut DNA is what allows its sequence to be modified, eliminating or inserting new DNA.
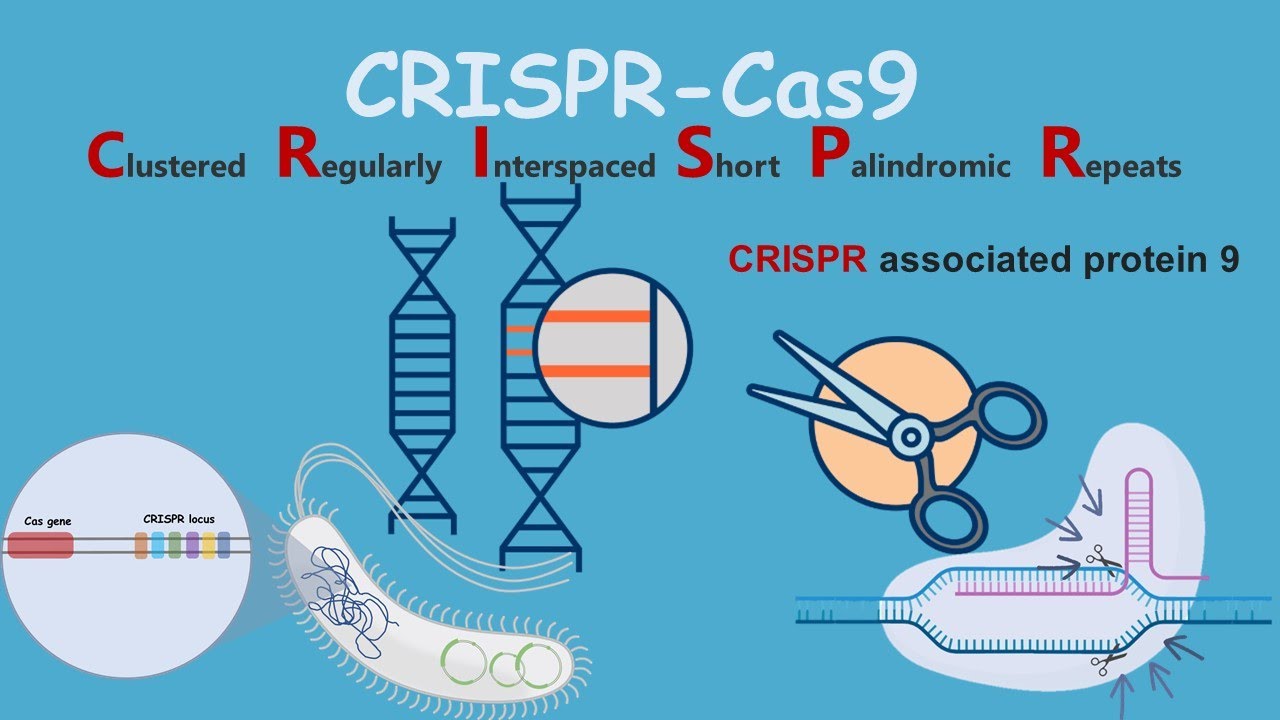
The acronym CRISPR/Cas9 comes from Clustered Regularly Interspaced Short Palindromic Repeats . The second is the name of a series of proteins, mainly nucleases, which were named after the CRISPR associated system (that is: «CRISPR-associated system»).
How did it come about?
It all started in 1987 when an article was published describing how some bacteria ( Streptococcus pyogenes) defended themselves against viral infections. These bacteria have enzymes that are capable of distinguishing between the genetic material of the bacteria and that of the virus and, once the distinction has been made, they destroy the genetic material of the virus.
However, the basis of this mechanism was not understood until later, when the genomes of some bacteria and other microorganisms were mapped. A certain area of the genome of many microorganisms, most notably archaea , was found to be filled with palindromic repeats (which read the same forwards and backwards) with no apparent function. These repeats were separated from each other by sequences called ‘spacers’ that resembled others in viruses and plasmids . Right in front of those repeats and “spacers” is a sequence called “leader”. These sequences are what were called CRISPR ( “Clustered Regularly Interspaced Short Palindromic Repeats”). Very close to this grouping could be found some genes that encoded for a type of nuclease: the cas genes .
When a virus enters the bacterium, it takes control of the cellular machinery and for this it interacts with different cellular components. But the bacteria that have this defense system have a complex made up of a Cas protein linked to RNA produced from the CRISPR sequences. Then the genetic material of the virus can interact with this complex. If that happens, the viral genetic material is inactivated and subsequently degraded. But the system goes further. Cas proteins are able to take a small part of the viral DNA, modify it and integrate it into the set of CRISPR sequences. That way, if that bacterium (or its offspring) encounters that same virus, it will now much more efficiently inactivate the viral genetic material. It is, therefore, a true bacterial immune system.
During subsequent years, research on this system continued, but it was not until 2012 that the key step was taken to convert this discovery, this biological observation, into a useful molecular tool in the laboratory. In August of this year, a team of researchers led by Drs Emmanuelle Charpentier at Umeå University and Jennifer Doudna at the University of California at Berkeley published an article in the journal Science demonstrating how to convert that natural machinery into a “programmable” editing tool, which was used to cut any strand of DNA in vitro. That is, they managed to program the system so that it would go to a specific position of any DNA (not just viral) and cut it.
The way in which they achieved it is too complex for what this blog intends. Suffice it to simply say that RNAs are used that direct the system towards the DNA to be cut.
How is DNA edited with this technology?
It all starts with the design of an RNA molecule (CRISPR or guide RNA) that is then going to be inserted into a cell. Once inside, it recognizes the exact site of the genome where the Cas9 enzyme should cut.
The process of editing a genome with CRISPR/Cas9 includes two stages. In the first layer, the guide RNA is associated with the enzyme Cas9. This guide RNA is specific to a specific DNA sequence, in such a way that by the rules of nucleotide complementarity it will hybridize in that sequence (the one we are interested in editing or correcting). Cas9 then acts, which is an endonuclease enzyme (that is, a protein that is capable of breaking a bond in the chain of nucleic acids), cutting the DNA. Basically we can say that the guide RNA acts as a guide dog, taking Cas9, the executor, to the site where it has to perform its function.
In the second stage, at least two natural repair mechanisms for broken DNA are activated. The first called indel (insertion-deletion) means that, after the cut site (the specific DNA sequence where the guide RNA was joined), either a hole appears in the chain, or another piece of chain is inserted. This leads to the loss of the original function of the cut DNA segment.
A second mechanism allows the incorporation of a specific sequence exactly at the original cleavage site. For this, logically, we have to give the cell the sequence that we want to integrate into the DNA.
Here is an explanatory video of the system:
problems you may have
In principle it has two technical drawbacks that have to be corrected (it is already in it). The first is derived from the fact that the specificity of the guide RNA is not complete. In other words, this RNA can hybridize, join with more than one site in the genome, which would lead the Cas9 enzyme to cut at a site that does not interest us. It must be remembered that the genome is made up of successions of four “letters”, A, T, G, and C, and the possibility of a certain sequence repeating itself is high, especially if the sequence is not very long (it is probable that , for example, the sequence AAATGGCAATC is in more than one gene. The probability of repetition decreases if the length of the “word” is increased). The second weak point of the technique is due to the fact that Cas9 can cut without the guide RNA being present. This is solved with more precise enzymes.
What is it good for?
In a molecular way we can say that this tool can be used to regulate gene expression, label specific sites of the genome in living cells, identify and modify gene functions and correct defective genes. It is also already being used to create animal models to study complex diseases such as schizophrenia, for which animal models did not previously exist.
How is life going to change us?
The possibilities are almost unimaginable. With CRISPR/Cas9 technology, a new era of genetic engineering is inaugurated in which the genome of any cell can be edited, corrected, altered in an easy, fast, cheap and, above all, highly precise way. Changing the genome means changing the essentials of a being, remember that.
In a relatively near future it will serve to cure diseases whose genetic cause is known and which until now were incurable. It is what you have surely heard many times as gene therapy. Work is already being done with this technology in these diseases such as Huntington’s Korea, Down Syndrome or sickle cell anemia. Another apparently futuristic application, but not so chimerical, is the reprogramming of our cells so that they cut the HIV genome.
Thus, MIT (Massachusetts Institute of Technology) announced in March 2014 that it had managed to cure an adult mouse of a liver disease ( tyrosinemia type I ) of genetic origin using this technology.
And, yes, as many of you are thinking, this technique is also valid for modifying the genomes of human embryos. Many books would have to be written on the ethical and social implications, but it is not our object here.
In addition to these health implications, it can also be used to improve transgenic foods (develop new varieties of plants and animals with specific genetic characteristics), modify bacteria and other microorganisms for industrial and food use.
patent war
Science also has an “ugly” side. And on this occasion, as almost always, it is derived from the (obvious) economic interests of CRISPR/Cas9 technology.
Shortly after Doudna and Charpentier’s famous paper, in January 2013 the labs of George Church at Harvard and Feng Zhang at MIT’s Broad Institute were the first to publish papers showing that CRISPR/Cas9 worked for human cells. Doudna independently published her own just a few weeks later.
In April 2014 Zhang and the Broad Institute were awarded the first of several general patents covering the use of CRISPR in eukaryotes . That gave them the rights to use CRISPR on mice, pigs, humans… pretty much any creature that wasn’t bacteria.
The speed of obtaining the patent surprised some. And it was because the Broad Institute had discreetly paid to have it reviewed very quickly, in less than six months. In addition, the process was carried out in an almost “secret” way. Along with the patent came more than a thousand pages of documents. Doudna had previously presented than Zhang’s, a patent application. But, according to Zhang, Doudna’s prediction in his application that his discovery would work in humans was “mere conjecture” and that instead he was the first to prove it in a different and “surprising” act of invention. To show that he was “the first to invent” the use of CRISPR-Cas in human cells, Zhang presented photos of lab notebooks that he says show he had the system up and running in early 2012, even before Doudna and Charpentier published their results or apply for their own patent. That timeline would mean that he discovered the CRISPR-Cas system independently. In an interview, Zhang claimed that he had made the discoveries on his own. Asked what he had learned from the Doudna and Charpentier article, he said “not much.”
On the other hand, Doudna and Charpentier’s lawyers are not going to sit idly by and are expected to mount an “interference proceeding” in the United States, which is a winner-takes-all legal proceeding and in which that one inventor can get hold of another’s patent.
Three start-ups are involved in the patent game for which the control of these patents is key. Those companies include Editas Medicine , Intellia Therapeutics , both of Cambridge, and CRISPR Therapeutics , a Basel, Switzerland , start-up co-founded by Charpentier. Zhang co-founded Editas Medicine, which in December 2014 announced that it had licensed the use of his patent from the Broad Institute. But Editas doesn’t have a monopoly on CRISPR because Doudna was also a co-founder of the company .. And since Zhang’s patent came out, Doudna has broken with the company, and his intellectual property in the form of his own pending patent has been licensed to Intellia. To further complicate matters, Charpentier sold his rights to the same patent application to CRISPR Therapeutics.
On the other hand, there are more and more voices asking that, due to the great capacity of CRISPR-Cas9 to cure diseases, the technology should not be protected by patent and be left as public access. Charpentier herself assures that the technology has been made freely available to the research community, so she does not believe that the patent poses any obstacle to scientific progress.
We will see what the future holds for us.